Issue # 1: First case report of feline Leishmaniosis in Galicia (Northwest Spain)
Issue # 1: 🚨 First Case of Feline Leishmaniasis in Galicia! 🐾 Our PCR analysis confirmed Leishmania infantum in a bone marrow sample from a cat—the first documented case in Galicia presenting cutaneous and ocular symptoms. Stray cats in Ourense may play a role in maintaining the parasite cycle in peri-urban and urban areas, highlighting the importance of monitoring and control efforts. #Leishmaniasis #FelineHealth #VeterinaryResearch #Galicia
María Isabel Silva-Torres, Judith Combarros-Leal, Alfonso Alba-Menéndez, Severino González-Roces
5/8/202416 min read
Abstract
Feline leishmaniosis (FeL) is an emerging infectious disease in cats, particularly in regions where Leishmania infantum is endemic. To the best of our knowledge, this is the first documented case of FeL in Galicia, Spain. The patient was a stray male cat that tested positive for both feline immunodeficiency virus (FIV) and feline leukemia virus (FeLV). The cat presented with cutaneous lesions, including interdigital swelling and a nodule on the upper right eyelid, accompanied by ocular damage. Cytological and histological examinations confirmed the presence of Leishmania spp., while polymerase chain reaction (PCR) analysis of a bone marrow sample detected L. infantum DNA. This case highlights the potential circulation of Leishmania among the local feline population, especially in immunocompromised cats, and underscores the importance of considering FeL in the differential diagnosis of cats with compatible clinical signs. Further studies are warranted to assess the epidemiological status of FeL in Galicia and its impact on feline health.
1. Introduction
Leishmaniosis is a neglected zoonotic parasitic disease caused in Europe by the protozoan Leishmania infantum and transmitted by the bite of female diptera of the genus Phlebotomus spp. In Spain, the species Phlebotomus perniciosus and Phlebotomus ariasi are the main vectors responsible for the transmission of this parasite [1], which affects both animals and humans and causes two clinical manifestations: cutaneous leishmaniosis (CL) or visceral leishmaniosis (VL). Domestic dogs (Canis familiaris) are the primary or main reservoir hosts, although the parasite has been isolated from several warm-blooded animals, both domestic and wild [2-4], such as ferrets [5] and lagomorphs [6]. However, there is evidence that felines (Felis catus domesticus) may play an important role in L. infantum epidemiology, but their categorization as primary or secondary reservoir hosts remains controversial [7].
Natural cases of feline leishmaniosis (FeL) in Europe have been reported in Mediterranean countries where canine leishmaniosis is endemic [8], but despite the increase in the number of cases in the last decades, probably due to veterinary practitioners awareness, there is still scarce information about its real prevalence since subclinical infections are common and clinical signs such as cutaneous lesions are mostly associated with immunosuppressed conditions as Feline immunodeficiency virus (FIV), Feline leukemia virus (FeLV) or cancer [9].
The epidemiological situation regarding FeL in Spain is yet under study, but some research shows that seroprevalences vary between geographical regions. Results reveal high seroprevalences in stray cats from the Balearic Islands [10] and Southern Spain [11], 13.2% and 28.3% respectively; while percentages of antibodies against L. infantum are lower in central (3,2%) and north-eastern areas (8,5%) [12, 13]. On the other hand, sporadic reports of visceral, cutaneous and ocular feline leishmaniosis have been described in the Mediterranean Basin [14-16] and southern territories [17]. In either case, both the higher percentage of Leishmania-infected cats and the diagnosis of clinical cases overlaps with the areas of highest seroprevalence for canine leishmaniosis [18]. However, clinical cases are not as abundant as in dogs probably due to underdiagnosis and feline natural resistance [9].
By contrast, northern Spain has traditionally been considered non-endemic for leishmaniosis, but studies reflect a change of perspective [19-22]. A good example is the Autonomous Community of Galicia (northwest Spain) where previous research has documented that the seroprevalence of L. infantum in dogs is as high as 35%, even higher than in other areas considered endemic [19,22,23] and two cutaneous human cases were reported in the last decade [24]. Despite this fact, to date there are no studies of feline seroprevalence in the region and there is no evidence of clinical cases. Therefore, the present article describes, for the first time, naturally occurring and autochthonous clinical case of L. infantum infection in a stray cat in the Galician city of Ourense.
2.Materials and methods
2.1 Area of study
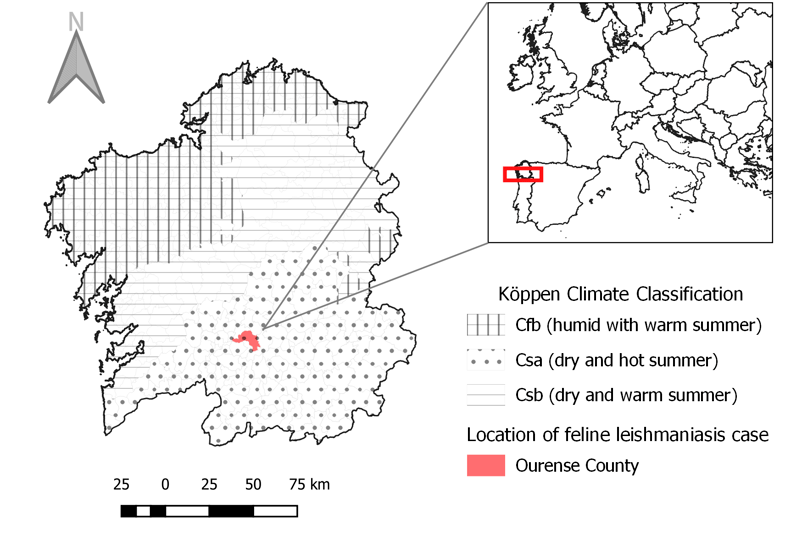
The case report presented was diagnosed in Ourense city, located in the South of Galicia (Fig.1). According to Köppen climate classification Galician inland territory presents a Csb weather (dry weather with warm summers) [25]. Nevertheless, within this territory, Ourense can be considered of Csa type (Mediterranean weather) [26], where summers are hot and dry with mean temperatures above 22°C [27].
2.2. Case history
An animal shelter referred a 6-year-old stray male cat of European shorthair to CatroGatos veterinary clinic during July of 2020. The animal physical aspect was not favourable, with suboptimal body condition and a rough, dull coat. The animal exhibited poor body condition and a rough, dull coat. A clinical examination revealed bilateral corneal opacities and vascularisation, initially suggesting a diagnosis of herpetic keratitis (Fig. 2A). A nodule measuring approximately 0.5 cm in diameter, solid in consistency and well-demarcated was detected in the upper right eyelid. Additionally, interdigital swelling on the left forelimb was observed. The clinical signs and absent medical history did not provide sufficient information to diagnose the case, needing further testing. A blood sample was collected for haematology and biochemistry, and tests for FIV and FeLV were conducted to determine the immune status. Needle aspiration of the interdigital lesion was performed for cytology, and the eyelid nodule was surgically excised and sent to an external laboratory for diagnosis. Finally, a bone marrow sample was extracted and sent to the laboratory "Instituto de Estudios Celulares y Moleculares (ICM)" to complete the molecular diagnosis.
2.3. PCR
DNA was isolated from bone marrow following QIAsymphony DSP DNA MiniKit® (QIAGEN, Germany) manufacturer’s instructions. The concentration of the extracted DNA was evaluated using the Qubit 4 fluorometer and subsequently adjusted to 20 µg/mL. A PCR protocol was chosen to detect the Leishmania at a specific level, using the pair of primers for Leishmania donovani complex: direct primer (5’- GTGGATAACGGCTCACATAACG -3’) reverse primer (3’- AATATGCGCACAACACAAACAC 5’). The temperature profile consisted of an initial denaturation step at 95ºC for 2 min, followed by 40 cycles of 30 s at 95ºC (denaturation), 30 s at 60ºC (annealing) and 30 s at 72ºC. Thermal processing was completed with a final extension step of 5 min at 72ºC. The amplification product of the specific PCR assay was a 311 bp DNA fragment.
The specificity of the DNA amplification was confirmed by Sanger ADN sequencing. Analysis of PCR samples was performed on both strands using the BigDye®Terminator Cycle Sequencing Kit and PRISM®377 DNA Sequencer (Applied Biosystems, FosterCity, California, USA). The resulting amplified products were then analysed and compared with the sequences deposited in the GenBank database using the Basic Local Alignment Search Tool (Standard Nucleotide BLAST) of the National Center for Biotechnology Information (NCBI).
3.Results
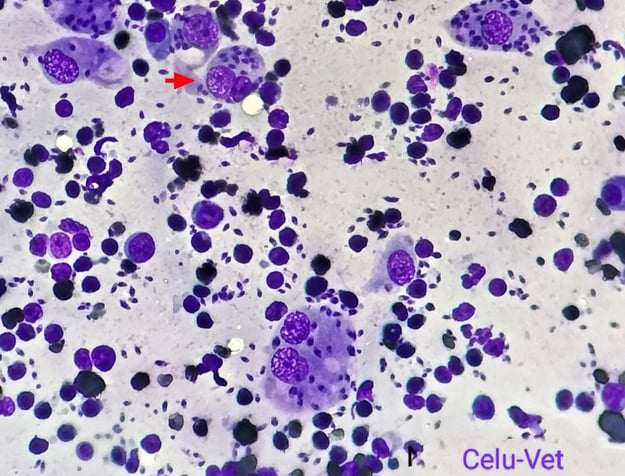
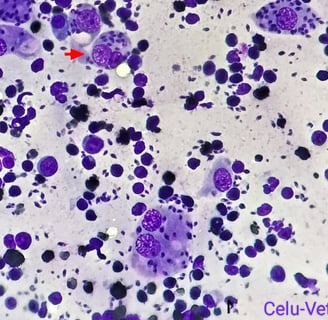
Blood tests revealed low haemoglobin (Hgb: 8.2 g/dL, reference range 12-18 g/dL) and haematocrit (PVC: 25.4%, reference range 37-55%). Serum biochemistry profiles were found to be within normal parameters; however serological tests for FIV and FeLV were positive. The interdigital sample cytology confirmed the presence of Leishmania spp. amastigotes (Fig. 2B). The eyelid nodule biopsy revealed an inflammatory infiltrate mainly composed of macrophages with Leishmania spp. amastigotes, plasmatic cells and to a lesser extent neutrophils and lymphocytes. The final diagnosis for this sample was therefore a chronic granulomatous dermatitis with intralesional presence of the parasite. Finally, PCR amplification for the bone marrow sample products showed 100% sequence homology to L. infantum.
The treatment was prescribed based on the physical examination and the results. For the resolution of keratitis and due to the challenges in handling the animal, a long-acting cyclosporine subconjunctival implant commonly used to treat ophthalmological conditions in equids, was chosen as a treatment option (https://cvm.ncsu.edu/nc-state-vet-hospital/equine/ophthalmology/recurrent-uveitis/). The eye implant was fitted to the patient under sedation and after a short period of time the improvement was noticeable. The treatment regimen was completed with antileishmanial therapy beginning with Milteforan ® (2mg/kg PO q 24h for 28 days) and changed to allopurinol (10 mg/kg PO q 12h for long-term). The latter treatment was successful in reducing the swelling of the paw and the improvement in corneal opacity was evident.
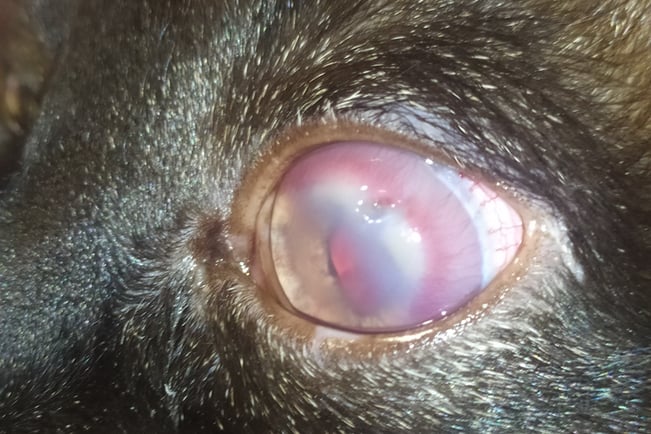
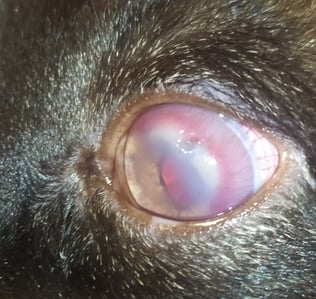
Fig. 2. A) Corneal inflammation an opacity in the patient.
Fig 1. Geographical location of the FeL case and Köppen climatic classification for Galicia
4.Discussion
Research and identification of feline leishmaniosis cases have increased over the years following the global trend [28]. However, there is still scarce information about what is happening in certain areas of Spain. Serological studies conducted in southern Galicia have revealed high antibodies against L. infantum in dogs, thereby classifying this region as hyperendemic for canine leishmaniasis [19, 22, 23]. Additionally, this area has documented cases of autochthonous human leishmaniasis [24]. This phenomenon can be attributed to the unique climatic conditions in southern Galicia, classified as a Mediterranean climate (climate type Csa Köppen), which are favourable for the transmission and survival of L. infantum [22]. Previous entomological monitoring has revealed the presence of the sandfly species P. perniciosus and P. ariasi in this region during specific weather conditions [19, 29]. A preliminary study on the phenology of these vectors has indicated that their activity starts in June and persists until late summer [29]. However, despite documented cases in dogs and humans, there has been no complementary research on how cats are affected in this region. To the best of our knowledge, this is the first clinical case of FeL recorded in the northwest of Spain.
The feline was infected in an urban environment in Ourense city, located in the Csa climate region (Fig. 1). It is suspected that the animal had not received veterinary care or preventive antiparasitic measures. Based on physical examination and the patient's immunocompromised status, an initial hypothesis suggested that the ocular damage could be associated with a viral infection. However, due to the presence of skin lesions, and the knowledge that Ourense is an endemic area for L. infantum, leishmaniosis was considered a differential diagnosis.
The clinical features of FeL are not pathognomonic and often non-specific symptoms. The most common manifestation involves cutaneous signs, particularly nodular dermatitis on the eyelids [8, 30]. A correlation between ocular lesions and the disease has also been documented [16, 31, 32]. Anaemia, as indicated by blood tests, is a non-specific finding. Although it has limited diagnostic value, it is among the expected haemogram abnormalities in affected cats [8]. However, immunosuppression induced by FeLV and FIV viruses has been demonstrated to promote parasite replication [11]. Furthermore, numerous studies have indicated that retrovirus coinfection increases the probability of clinical signs in cats [9], a finding that is in accordance with the patient's clinical history.
Cytological, histological, and molecular methods were used to confirm the final diagnosis.The first two were chosen because they are relatively rapid and low cost routinary diagnostic tools in veterinary practice [16]. The molecular PCR technique was implemented because of its high sensitivity and specificity and for the parasite species characterization [8]. No other laboratory tests were included due to the financial constraints of the animal caretakers and the consideration that the above mentioned are valid techniques for FeL diagnosis [8, 9].
Anti-leishmanial treatment of clinical cases is not uncommon, but there are still no conclusive studies on the true efficacy and the therapeutic approach used for dogs is usually extrapolated. Allopurinol administration is usually considered the best option [28,32,33]. The use of miltefosine in cats is included among the possibilities and although further research is needed to explore possible side effects [32, 34], Milteforan ® initial choice was made based on difficult patient handling and easiness of administration. However, it was finally necessary to switch to long-term allopurinol. Oral administration together with the long-acting cyclosporine subconjunctival implant had favourable results as clinical signs improved, although regular follow-up is necessary to check progress.
Knowledge of FeL cases is relevant from a One Health perspective [35], as felines are a source of infection for sandflies [36, 37]. This can be a problem, especially in the stray cat population, where protective measures against these vectors are often lacking and veterinary care is limited. Thus, urban and peri-urban cycles may be perpetuated, increasing the risk of infection of other mammals, including humans [3]. In this sense, evidence of this first autochthonous FeL case in Galicia is important from an animal and public health point of view, not only locally but also internationally. At the local level, it contributes to raising clinicians' awareness of the possible circulation of the parasite in the indigenous cat population and, consequently, to better management of future cases, avoiding underdiagnosis in patients with compatible clinical manifestations. In addition, other measures to prevent and reduce the source of infection must complement the detection and treatment of Leishmania-positive cats. Some useful recommendations, not only for indigenous cats but also for incoming pets, include implementing protective measures to avoid phlebotomine bites, such as keeping cats indoors during the vector season or using insecticides when possible [9,33,38]. On the other hand, in Europe [35], human and animal leishmaniasis are mistakenly considered a local rather than a transboundary problem, and the uniform reporting of cases is still a challenge. Non-reporting may pose a risk, especially if infected hosts are moved to other non-endemic areas. In this regard, this is not the first time that cats exported from Spain have been diagnosed with feline leishmaniasis in the country of destination [39, 40].
Therefore, we want to contribute to the knowledge of clinical cases of FeL in Spain, to highlight the inclusion of this vector-borne disease as a differential diagnosis in Galician endemic areas. Additionally, we highlight the importance of preventive measures, particularly for outdoor cats, which are at higher risk of exposure. Incorporating serological and molecular surveillance techniques is advisable when adopting locally rescued cats or pets that have traveled to Galicia, especially in cases where exposure to infected sandflies is suspected [33]. Finally, further research is essential to obtain comprehensive epidemiological data on the native cat population, as this is crucial for local disease control and for mitigating risks to both animal and public health.
Spanish original version in Multimética Ediciones Veterinarias journal
Fig. 2.B) Cytological image of left forelimb interdigital swelling with presence of Leishmania spp. amastigotes in macrophages (red arrow)
Acknowledgements
This work would not have been possible without the help and resources of the ICM laboratory, and the interest of the veterinarians involved in the case; a special mention to ophthalmologist Rubén Ripplinger (Oculus Vet). The authors would also like to thank Mamen Rastrilla Calleja (CeluVet) for kindly donating the cytological photos. Finally, our sincere gratitude to the animal protection organisation (Asociación Protectora Patrulla Callejera) for the great work they do.
Ethical statement
In the development of this case, non-experimental animals or techniques were used, however, recognised high standards (‘best practice’) of individual veterinary clinical patient care were implemented. Therefore, approval by an ethics committee was not necessary.
REFERENCES
[1] J. Lucientes, J. A. Castillo, M. J. Gracia, M. À. Peribáñez MÁ, Flebotomos, de la biología al control, Rev. Electron. de Vet. VI (2005) 1–8.
[2] T. D. Souza, A. P. Turchetti, R. T. Fujiwara, T. A. Paixão, R. L. Santos, Visceral leishmaniasis in zoo and wildlife, Vet Parasitol. 200 (2014) 233–241, https://doi.org/10.1016/j.vetpar.2013.12.025
[3] M. M. Alcover, A. Ribas , M. C. Guillén, D. Berenguer, M. Tomás-Pérez, C. Riera, R. Fisa, Wild mammals as potential silent reservoirs of Leishmania infantum in a Mediterranean area, Prev. Vet. Med. 175 (2020), 104874, https://doi.org/10.1016/j.prevetmed.2019.104874
[4] L. Cardoso, H. Schallig, M. F. Persichetti, M. G. Pennisi, New epidemiological aspects of animal Leishmaniosis in Europe: the role of vertebrate hosts other than dogs, Pathogens 10 (2021), 307, https://doi.org/10.3390/pathogens10030307
[5] J. Giner, A. Basurco, M. M. Alcover, C. Riera, R. Fisa, R. A. Lópe, C. Juan-Sallés, M. T. Verde, A. Fernández, A. Yzuel, S. Villanueva-Saz, First report on natural infection with Leishmania infantum in a domestic ferret (Mustela putorius furo) in Spain, Vet. Parasitol. Reg. Stud. Rep. 19 (2020), 100369, https://doi.org/10.1016/j.vprsr.2020.100369
[6] N. García, I. Moreno, J. Alvarez, M. L. De la Cruz, A. Navarro, M. Pérez-Sancho, T. García-Seco, A. Rodríguez-Bertos, M. L. Conty, A. Toraño, A. Prieto, L. Domínguez, M. Domínguez, Evidence of Leishmania infantum infection in rabbits (Oryctolagus cuniculus) in a natural area in Madrid, Spain, Biomed. Res. Int. 2014 (2014), 1–5, https://doi.org/10.1155/2014/318254
[7] S. Asfaram, M. Fakhar, S.H. Teshnizi, Is the cat an important reservoir host for visceral leishmaniasis? A systematic review with meta-analysis. J. Venom. Anim. Toxins. Incl. Trop. Dis. 25 (2019), 1–10, https://doi.org/10.1590/1678-9199-jvatitd-2019-0012.
[8] A. Pereira, C. Maia, Leishmania infection in cats and feline leishmaniosis: an updated review with a proposal of a diagnosis algorithm and prevention guidelines, CRPVBD 1 (2021), 100035, https://doi.org/10.1016/j.crpvbd.2021.100035.
[9] M. G. Pennisi, L. Cardoso, G. Baneth, P. Bourdeau, A. Koutinas, G. Miró, G. Oliva, L. Solano-Gallego, LeishVet update and recommendations on feline leishmaniosis, Parasit Vectors 8 (2015), 302, https://doi.org/10.1186/s13071-015-0909-z.
[10] K. Sherry, G. Miró, M. Trotta, C. Miranda, A. Montoya, C. Espinosa, F. Ribas, T. Furlanello, L. Solano-Gallego, A serological and molecular study of Leishmania infantum infection in cats from the island of Ibiza (Spain), Vector-Borne Zoonotic. Dis 11 (2011), 239–245, https://doi.org/10.1089/vbz.2009.0251.
[11] J. Martín-Sánchez, C. Acedo, M. Muñoz-Pérez, B. Pesson, O. Marchal, F. Morillas-Márquez, Infection by Leishmania infantum in cats: epidemiological study in Spain, Vet. Parasitol. 145 (2007), 267–273, https://doi.org/10.1016/j.vetpar.2006.11.005.
[12] G. Miró, C. Rupérez, R. Checa, R. Gálvez, L. Hernández, M. García, I. Canorea, V. Marino, A. Montoya, Current status of L. infantum infection in stray cats in the Madrid region (Spain): implications for the recent outbreak of human leishmaniosis?, Parasit. Vectors 7 (2014), 112, https://doi.org/10.1186/1756-3305-7-112.
[13] M. M. Alcover, A. Basurco, A. Fernandez, C. Riera, R. Fisa, A. Gonzalez, M. Verde, A. M. Garrido, H. Ruíz, A. Yzuel, S. Villanueva-Saz, A cross-sectional study of Leishmania infantum infection in stray cats in the city of Zaragoza (Spain) using serology and PCR, Parasit. Vectors 14 (2021), 178, https://doi.org/10.1186/s13071-021-04682-w.
[14] M. Leiva, A. Lloret, T. Peña, X. Roura, Therapy of ocular and visceral leishmaniasis in a cat, Vet. Ophthalmol. 8 (2005), 71–75, https://doi.org/10.1111/j.1463-5224.2005.00342.x.
[15] A. Dalmau, M. Osso, A. Oliva, L. Anglada, X. Sarobé, E. Vives, Leishmaniosis felina a propósito de un caso clínico. ¿Nos olvidamos de que existe?, Clin. Vet. Peq. Anim. 28 (2008), 233–237.
[16] A. Fernandez-Gallego, L. Feo Bernabe, A. Dalmau, D. Esteban-Saltiveri, A. Font, M. Leiva, A. Ortuñez-Navarro, M. T. Peña, M. D. Tabar, L. Real-Sampietro, F. Saló, A. Lloret, M. Bardagí, Feline leishmaniosis: diagnosis, treatment and outcome in 16 cats, JFMS 22 (2020), 993–1007, https://doi.org/10.1177/1098612X20902865
[17] J. Hervás, F. Chacón-Manrique De Lara, J. López, J. C. Gómez-Villamandos, M. J. Guerrero, A, Moreno, Granulomatous (pseudotumoral) iridociclitis associated with leishmaniasis in a cat, Vet. Rec. 149 (2001), 624–625, https://doi.org/10.1136/vr.149.20.624.
[18] J. A. Montoya-Alonso, R. Morchón, N. Costa-Rodríguez, J. I. Matos, Y. Falcón-Cordón, E. Carretón, Current distribution of selected vector-borne diseases in dogs in Spain, Front. Vet. Sci. 7 (2020), 1–11, https://doi.org/10.3389/fvets.2020.564429.
[19] G. Miró, R. Checa, A. Montoya, L. Hernández, D. Dado, R. Gálvez, Current situation of Leishmania infantum infection in shelter dogs in northern Spain, Parasit. Vectors 5 (2012), 60, https://doi.org/10.1186/1756-3305-5-60.
[20] L. Del Río, L. Chitimia, A. Cubas, I. Victoriano, P. De la Rúa, X. Gerrikagoitia, M. Barral, C. I. Muñoz-García, E. Goyena, D. García-Martínez, R. Fisa, C. Riera, L. Murcia, M. Segovia, E. Berriatua, Evidence for widespread Leishmania infantum infection among wild carnivores in L. infantum periendemic northern Spain, Prev. Vet. Med. 113 (2014), 430–435, https://doi.org/10.1016/j.prevetmed.2013.12.001
[21] A. Oleaga, S. Zanet, A. Espí, M. R. Pegoraro de Macedo, C. Gortázar, E. Ferroglio, Leishmania in wolves in northern Spain: A spreading zoonosis evidenced by wildlife sanitary surveillance, Vet. Parasitol. 255 (2018), 26–31, https://doi.org/10.1016/j.vetpar.2018.03.015
[22] R. Gálvez, A. Montoya, I. Cruz, C. Fernández, O. Martín, R. Checa, C. Chicharro, S. Migueláñez, V. Mariano, G. Miró, Latest trends in Leishmania infantum infection in dogs in Spain, Part I: Mapped seroprevalence and sand fly distributions, Parasit. Vectors 13 (2020), 204. https://doi.org/10.1186/s13071-020-04081-7.
[23] I. Amusategui, A. Sainz, E. Aguirre, M. A. Tesouro, Seroprevalence of Leishmaniasis infantum in Northwest Spain, an area traditionally considered free of leishmaniasis, Ann. N. Y. Acad. Sci. 1026 (2004), 154–157, https://doi.org/10.1196/annals.1307.022
[24] RENAVE. La leishmaniasis en España: evolución de los casos notificados en la Red Nacional de Vigilancia Epidemiológica desde 2005 a 2016 y resultados de la vigilancia de 2014 a 2017, Boletín Epidemiológico Semanal, 27 (2019), 27.
[25] AEMET (2018). Mapas climáticos de España (1981-2010) y ETo (1996-2016). In Agencia Estatal de Meteorología. Ministerio para la Transición Ecológica.
[26] Concello da Cultura Gallega. (2008). Historia da meteoroloxía e da climatoloxía de Galicia. Retrieved from http://books.google.es/books?id=FF0QuOQ3AwoC.
[27] M. C. Peel, B. L. Finlayson, T. A. McMahon, Updated world map of the Köppen-Geiger climate classification, Hydrol. Earth Syst. Sci. 11 (2007), 1633–1644. https://doi.org/10.5194/hess-11-1633-2007
[28] M. G. Pennisi, M. F. Persichett, Feline leishmaniosis: Is the cat a small dog?, Vet. Parasitol. 251 (2018), 131–137, https://doi.org/10.1016/j.vetpar.2018.01.012
[29] M. I. Silva-Torres, Epidemiología de dípteros que actúan como vectores de zoonosis en Galicia. Diseño de una red de vigilancia de vectores. University of Santiago de Compostela (2021).
[30] F. Abramo, F. Albanese, S. Gattuso, A. Randone, I. Fileccia, C. Dedola, F. Ibba, P. Ottaiano, E. Brianti, Skin lesions in feline leishmaniosis: a systematic review, Pathogens 10 (2021) , 472. https:// doi.org/10.3390/pathogens10040472.
[31] J. A. Navarro, J. Sánchez, C. Peñafiel-Verdú, A. J. Buendía, J. Altimira, M. Vilafranca, Histopathological lesions in 15 cats with Leishmaniosis, J. Comp. Pathol. 143 (2010), 297–302, https://doi.org/10.1016/j.jcpa.2010.03.003.
[32] M. García-Torres, M. C. López, S. Tasker, M. R. Lappin, C. Blasi-Brugué, X. Roura, Review and statistical analysis of clinical management of feline leishmaniosis caused by Leishmania infantum, Parasit. Vectors 15 (2022), 253, https://doi.org/10.1186/s13071-022-05369-6.
[33] R. Rocha, A. Pereira, C. Maia, A global perspective on non-autochthonous canine and feline Leishmania infection and leishmaniosis in the 21st century, Acta Trop. 237 (2023), 106710, https://doi.org/10.1016/j.actatropica.2022.106710
[34] R. O. Leal, H. Pereira, C. Cartaxeiro, E. Delgado, M. C. Peleteiro, I. Pereira da Fonseca, Granulomatous rhinitis secondary to feline leishmaniosis: report of an unusual presentation and therapeutic complications, J. Feline Med. Surg. Open Rep. 4 (2018), 205511691881137. https://doi.org/10.1177/2055116918811374
[35] E. Berriatua, C. Maia, C. Conceição, Y. Özbel, S. Töz, G. Baneth, P. Pérez-Cutillas, M. Ortuño, C. Muñoz, Z. Jumakanova, A. Pereira, R. Rocha, B. Monge-Maillo, E. Gasimov, Y. Van der Stede, G. Torres, C. M. Gossner, Leishmaniases in the European Union and neighboring countries, Emerg. Infect. Dis. 27 (2021), 1723–1727, https://doi.org/10.3201/eid2706.210239
[36] M. De Colmenares, M. Portús, J. Botet, C. Dobaño, M. Gállego, M. Wolff, G. Seguí, Identification of blood meals of Phlebotomus perniciosus (Diptera: Psychodidae) in Spain by a competitive enzyme-linked immunosorbent assay biotin/avidin method, J. Med. Entomol. 32 (1995), 229–233, https://doi.org/10.1093/jmedent/32.3.229.
[37] A. Pereira, J. M. Cristóvão, H. Vilhena, Â. Martins, P. Cachola, J. Henriques, M. Coimbra, A. Catarino, T. Lestinova, T. Spitzova, P. Volf, L. Campino, C. Maia, Antibody response to Phlebotomus perniciosus saliva in cats naturally exposed to phlebotomine sand flies is positively associated with Leishmania infection, Parasit. Vectors 12 (2019), 128, https://doi.org/10.1186/s13071-019-3376-0
[38] A. E. Ahuir-Baraja, M. P. Ruiz, M. M. Garijo, L. Llobat, Feline Leishmaniosis: an emerging public health problem, Vet. Sci. 8 (2021), 173, https://doi.org/10.3390/vetsci8090173
[39] S. Rüfenacht, H. Sager, N. Müller, V. Schaerer, A. Heier, M. M. Welle, P. J. Roosje, Two cases of feline leishmaniosis in Switzerland, Vet. Rec. 2005, 542–545, https://doi.org/10.1136/vr.156.17.542
[40] I. Schäfer, B. Kohn, M. Volkmann, E. Müller, Retrospective evaluation of vector-borne pathogens in cats living in Germany (2012–2020), Parasit. Vectors 14 (2021), 123, https://doi.org/10.1186/s13071-021-04628-2